Why Does Chlorine Displace Bromine From Sodium Bromide
Cost Comparison of Bromine vs. Chlorine is more reactive than bromine you know this because chlorine is above bromine in group 7.

Chemical Test For Chlorine 2 Chlorine Chemical Ccls
The solution turns brown.

. Because chlorine is more reactive than bromine it displaces bromine from sodium bromide. Because chlorine is more reactive than bromine it displaces bromine from sodium bromide. When chlorine as a gas or dissolved in water is added to sodium bromide solution the chlorine takes the place of the bromine.
When chlorine as a gas or dissolved in water is added to sodium bromide solution the chlorine takes the place of the bromine. This brown colour is the displaced bromine. When chlorine as a gas or dissolved in water is added to sodium bromide solution the chlorine takes the place of the bromine.
Compared to bromine chlorine requires much more maintenance. Sodium bromide is a salt of bromine that will dissolve in water. Helpful 0 Not Helpful 0 Add a Comment.
Because chlorine is more reactive than bromine it displaces. The solution turns brown. The chlorine has gone to form sodium chloride.
Bromine also has a greater efficiency over a wider pH range than chlorine. Im aware that the concentration affects the electrode potentials but the question simply states an aqueous solution of NaBr with no reference to concentration whatsoever so I can only assume standard solutions. The only difference is that technically bromine is 225 times heavier than chlorine which is why the test kits have dual scales with bromine twice as high as chlorine.
The chlorine has gone to form sodium chloride. Because chlorine is more reactive than bromine it displaces bromine from sodium bromide. Chlorine atoms have substantially greater electronegativity than bromine atoms while chloride and bromide ions both have about the same very low electronegativity.
As the electrode potential for bromine is higher it means bromine should be reduced rather than ceOH- ions. When chlorine as a gas or dissolved in water is added to sodium bromide solution the chlorine takes the place of the bromine. Bromine and chlorine will sanitize and oxidize pool or spa water but bromine works better at higher temperatures and is softer on the skin whereas chlorine is cheaper works for longer and doesnt break down as quickly in ultraviolet light.
One of the main reasons most pool owners opt for chlorine as a. If you measure a zero bromine reading that just means there is no bromine or chlorine but it does not mean there is no bromide. The chlorine has gone to form sodium chloride.
This means that if chlorine as a gas or dissolved in water is added to sodium bromide solution bromine forms and the mixture turns brown. This brown colour is the displaced bromine. So chlorine will displace bromine from sodium bromide solution.
Chlorine is a stronger oxidizing agent than bromine.

Chemical Test For Iodine Iodine Chemical Test
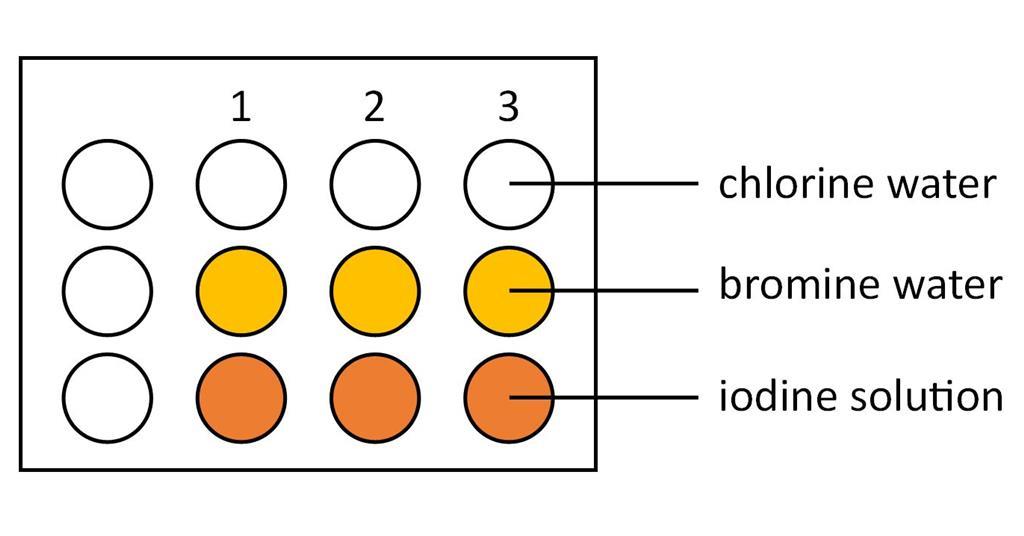
Halogens In Aqueous Solution And Their Displacement Reactions Experiment Rsc Education

Question Video Writing A Net Ionic Equation For The Reaction Of Chlorine Water With Aqueous Sodium Bromide Nagwa
No comments for "Why Does Chlorine Displace Bromine From Sodium Bromide"
Post a Comment